| |
 |
Mathiasite Mineral Data
|
|
|
General Mathiasite Information
|
 Chemical Formula: Chemical Formula: |
(K,Ca,Sr)(Ti,Cr,Fe,Mg)21O38 |
 Composition: Composition: |
Molecular Weight = 1,682.56 gm |
| |
Potassium 1.39 % K 1.68 % K2O |
| |
Strontium 0.52 % Sr 0.62 % SrO |
| |
Calcium 0.71 % Ca 1.00 % CaO |
| |
Magnesium 0.72 % Mg 1.20 % MgO |
| |
Titanium 36.99 % Ti 61.72 % TiO2 |
| |
Chromium 18.54 % Cr 24.25 % CrO |
| |
Iron 4.98 % Fe 6.41 % FeO |
| |
Oxygen 36.13 % O |
| |
______ ______ |
| |
100.00 % 96.86 % = TOTAL OXIDE |
 Empirical Formula: Empirical Formula: |
K0.6Ca0.3Sr0.1Ti13Cr6Fe2+1.5Mg0.5O38 |
 Environment: Environment: |
In kimberlite. |
 IMA Status: IMA Status: |
Approved IMA 1983 |
 Locality: Locality: |
Jagersfontein mine, Orange Free State, South Africa. Link to MinDat.org Location Data. |
 Name Origin: Name Origin: |
Named for Frances Celia Morna Mathias (1913-), British-South African geochemist, University of Capetown, South Africa. |
 Name Pronunciation: Name Pronunciation: |
Mathiasite + Pronunciation  |
 Synonym: Synonym: |
ICSD 35353 |
| |
PDF 46-1468 |
| |
Mathiasite Image |
 Images:
Images:
|
|
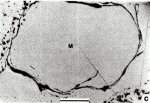
Mathiasite
Comments: Mathiasite (M) exhibiting typical curvilinear cracks and incipient decomposition (upper left and lower right). Sample JFIS-I, Jagersfontein. Reflected light in air. (AmMin, v68:494).
Location: Metasomatized peridotites and heavy media concentrates from four kimberlites in the Republic of South Africa.
Scale: Scale Bar 0.2 mm.
© American Mineralogist |
|
Mathiasite Crystallography
|
 Axial Ratios: Axial Ratios: |
a:c = 1:1.99324 |
 Cell Dimensions: Cell Dimensions: |
a = 10.36, c = 20.65, Z = 3; V = 1,919.42 Den(Calc)= 4.37 |
 Crystal System: Crystal System: |
Trigonal - Rhombohedral H-M Symbol ( 3) Space Group: R 3 |
 X Ray Diffraction: X Ray Diffraction: |
By Intensity(I/Io): 1.44(1), 2.14(1), 2.25(0.9), |
| |
Physical Properties of Mathiasite
|
 Cleavage: Cleavage: |
None |
 Color: Color: |
Black. |
 Density: Density: |
4.6 |
 Diaphaneity: Diaphaneity: |
Opaque |
 Fracture: Fracture: |
Conchoidal - Fractures developed in brittle materials characterized by smoothly curving surfaces, (e.g. quartz). |
 Hardness: Hardness: |
7.5 - Garnet |
 Luster: Luster: |
Metallic |
 Streak: Streak: |
gray |
| |
Optical Properties of Mathiasite
|
 Dichroism (e): Dichroism (e): |
dark tan. |
 Dichroism (w): Dichroism (w): |
weaker dark tan. |
 Optical Data: Optical Data: |
Uniaxial (?). |
| |
NCalc= 2.36 - from Gladstone-Dale relationship (KC = 0.3122) where Ncalc=Dcalc*KC+1
NCalc= 2.44 - from Gladstone-Dale relationship (KC = 0.3122) where Ncalc=Dmeas*KC+1
|
 RL Anisotrophism: RL Anisotrophism: |
Moderate, brownish tan to dark brown. |
 RL Color: RL Color: |
Tan. |
 Reflectivity Reflectivity |
Standardized Intensity (100%) Reflection Spectra of Mathiasite in Air
| λ |
R1 |
R2 |
 |
∑ R1(λ) |
∑ R2(λ) |
| 400 nm |
20.10 |
20.80 |

 |
|
|
| 420 nm |
19.50 |
20.20 |

 |
| 440 nm |
19.00 |
19.70 |

 |
| 460 nm |
18.50 |
19.20 |

 |
| 480 nm |
18.10 |
18.60 |

 |
| 500 nm |
17.70 |
18.40 |

 |
| 520 nm |
17.40 |
18.20 |

 |
| 540 nm |
17.20 |
17.90 |

 |
| 560 nm |
16.90 |
17.70 |

 |
| 580 nm |
16.80 |
17.60 |

 |
| 600 nm |
16.60 |
17.40 |

 |
| 620 nm |
16.50 |
17.40 |

 |
| 640 nm |
16.50 |
17.30 |

 |
| 660 nm |
16.50 |
17.30 |

 |
| 680 nm |
16.40 |
17.30 |

 |
| 700 nm |
16.40 |
17.30 |

 |
Calculated Relative Intensity Colors of Mathiasite in Air
Relative
Intensity |
0% |
60% |
100% |
120% |
180% |
240% |
300% |
360% |
420% |
480% |
520% |
| R1 |
|
|
|
|
|
|
|
|
|
|
|
| R2 |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Calculated Properties of Mathiasite
|
 Electron Density: Electron Density: |
Bulk Density (Electron Density)=4.16 gm/cc
note: Specific Gravity of Mathiasite =4.37 gm/cc.
|
 Fermion Index: Fermion Index: |
Fermion Index = 0.06
Boson Index = 0.94 |
 Photoelectric: Photoelectric: |
PEMathiasite = 12.77 barns/electron
U=PEMathiasite x relectron= 53.09 barns/cc.
|
 Radioactivity: Radioactivity: |
GRapi = 19.11 (Gamma Ray American Petroleum Institute Units)
Concentration of Mathiasite per GRapi unit = 5.23 (%)
Estimated Radioactivity from Mathiasite  - barely detectable - barely detectable
|
| |
Mathiasite Classification
|
 Dana Class: Dana Class: |
08.05.01.07 (08)Multiple Oxides with Nb, Ta, and Ti |
| |
(08.05)Dana Type |
| |
(08.05.01)Crichtonite group (ABC18 T2 O38) |
| |
08.05.01.01 Landauite NaMnZn2(Ti,Fe)6Ti12O38 R 3 3 |
| |
08.05.01.02 Loveringite (Ca,Ce)(Ti,Fe,Cr,Mg)21O38 R 3 3 |
| |
08.05.01.03 Crichtonite (Sr,La,Ce,Y)(Ti,Fe,Mn)21O38 R 3 3 |
| |
08.05.01.04 Senaite Pb(Ti,Fe,Mn)21O38 R 3 3 |
| |
08.05.01.05 Davidite-(La) (La,Ce,Ca)(Y,U)(Ti,Fe)20O38 R 3 3 |
| |
08.05.01.06 Davidite-(Ce) (Ce,La)(Y,U)(Ti,Fe)20O38 R 3 3 |
| |
08.05.01.07 Mathiasite (K,Ca,Sr)(Ti,Cr,Fe,Mg)21O38 R 3 3 |
| |
08.05.01.08 Lindsleyite (Ba,Sr)(Ti,Cr,Fe,Mg)21O38 R 3 3 |
| |
08.05.01.09 Dessauite! (Sr,Pb)(Y,U)(Ti,Fe)20O38 P 3 3 |
| |
08.05.01.10 Cleusonite! Pb(U,U)(Ti,Fe,Fe)20(O,OH)38 R 3 3 |
| |
08.05.01.11 Gramaccioliite-(Y)! (Pb,Sr)(Y,Mn)Fe2(Ti,Fe)18O38 R 3 3 |
 Strunz Class: Strunz Class: |
04.CC.40 04 - OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodate |
| |
04.C - Metal:Oxygen = 2:3, 3:5, and Similar |
| |
04.CC -With large and medium-sized cations |
| |
04.CC.40 Crichtonite (Sr,La,Ce,Y)(Ti,Fe,Mn)21O38 R 3 3 |
| |
04.CC.40 Dessauite! (Sr,Pb)(Y,U)(Ti,Fe)20O38 P 3 3 |
| |
04.CC.40 Davidite-(Ce) (Ce,La)(Y,U)(Ti,Fe)20O38 R 3 3 |
| |
04.CC.40 Davidite-(La) (La,Ce,Ca)(Y,U)(Ti,Fe)20O38 R 3 3 |
| |
04.CC.40 Mathiasite (K,Ca,Sr)(Ti,Cr,Fe,Mg)21O38 R 3 3 |
| |
04.CC.40 Lindsleyite (Ba,Sr)(Ti,Cr,Fe,Mg)21O38 R 3 3 |
| |
04.CC.40 Landauite NaMnZn2(Ti,Fe)6Ti12O38 R 3 3 |
| |
04.CC.40 Loveringite (Ca,Ce)(Ti,Fe,Cr,Mg)21O38 R 3 3 |
| |
04.CC.40 Senaite Pb(Ti,Fe,Mn)21O38 R 3 3 |
| |
04.CC.40 Cleusonite! Pb(U,U)(Ti,Fe,Fe)20(O,OH)38 R 3 3 |
| |
04.CC.40 Gramaccioliite-(Y)! (Pb,Sr)(Y,Mn)Fe2(Ti,Fe)18O38 R 3 3 |
| |
Other Mathiasite Information
|
 References: References: |
NAME( Dana8) PHYS. PROP.(Enc. of Minerals,2nd ed.,1990) OPTIC PROP.(AntBidBlaNic3) |
 See Also: See Also: |
Links to other databases for Mathiasite :
1 - Am. Min. Crystal Structure Database
2 - Athena
3 - EUROmin Project
4 - Ecole des Mines de Paris
5 - GeoScienceWorld
6 - Google Images
7 - Google Scholar
8 - Handbook of Mineralogy (MinSocAm)
9 - Handbook of Mineralogy (UofA)
10 - MinDAT
11 - Mineralienatlas (Deutsch)
12 - Online Mineral Museum
13 - QUT Mineral Atlas
14 - Ruff.Info
15 - WWW-MINCRYST
Search for Mathiasite using:
[AOL]
[Bing]
[Dog Pile]
[GeoScienceWorld]
[HotBot]
[Ixquick]
[Lycos]
[MAMMA]
[Scirus]
[Teoma]
[WebCrawler]
[Wikipedia]
[YAHOO]
Visit our Advertisers for Mathiasite :
Ask about Mathiasite here :
Ask-A-Mineralogist from the Mineralogical Society of America
Mindat.org's Discussion Groups
Original Rockhounds Discussion Group
Rockhounds Discussion Group on Yahoo Groups
Mineral Discussion Forum from Fabre Minerals - also available in
Espaņol
Print or Cut-and-Paste your Mathiasite Specimen Label here :
|
Mathiasite
(K,Ca,Sr)(Ti,Cr,Fe,Mg)21O38
Dana No: 08.05.01.07 Strunz No: 04.CC.40
Locality:
Notes:
|
|
| |
| |
|
| |
Dakota Matrix
Excalibur Minerals
Exceptional Minerals
Hudson Insitute
John Betts Fine Minerals
Mc Dougall Minerals
Mineral News
Rock and Mineral Shows
Weinrich Minerals, Inc.



 - barely detectable
- barely detectable