Keystoneite Mineral Data
General Keystoneite Information
Chemical Formula: Mg0.5[Ni++Fe++(TeO3)3]•4.5(H2O)
Composition: Molecular Weight = 734.55 gm
Magnesium 1.65 % Mg 2.74 % MgO
Iron 7.60 % Fe 9.78 % FeO
Nickel 7.99 % Ni 10.17 % NiO
Tellurium 52.11 % Te 65.18 % TeO2
Hydrogen 1.23 % H 11.04 % H2 O
Oxygen 29.40 % O
______ ______
100.00 % 98.91 % = TOTAL OXIDE
Empirical Formula: Mg0.5 NiFe2+ (TeO3 )3 •4.5(H2 O)
Environment: Secondary mineral in telluride-bearing ores.
IMA Status: Approved IMA 1988
Locality: Keystone mine, Magnolia distict, Boulder County, Colorado, USA. Link to MinDat.org Location Data.
Name Origin: Named for the locality
Name Pronunciation: Keystoneite + Pronunciation
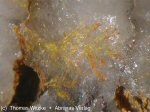
Keystoneite Image
Images:
Keystoneite Crystallography
Axial Ratios: a:c = 1:0.8141
Cell Dimensions: a = 9.344, c = 7.607, Z = 2; V = 575.19 Den(Calc)= 4.24
Crystal System: Hexagonal - Dipyramidal 3 /m
X Ray Diffraction: By Intensity(I/Io ): 2.77(1), 8.12(0.9), 4.05(0.8), 1.72(0.6), 1.498(0.5), 2.84(0.5), 2.95(0.5), 3.81(0.4),
Physical Properties of Keystoneite
Cleavage: None
Color: Golden yellow, Orange yellow.
Density: 4.4
Habit: Acicular - Occurs as needle-like crystals.
Habit: Radial - Crystals radiate from a center without producing stellar forms (e.g. stibnite)
Luster: Adamantine
Optical Properties of Keystoneite
Gladstone-Dale: CI meas = 0.006 (Superior) - where the CI = (1-KPDmeas /KC ) calc = -0.032 (Excellent) - where the CI = (1-KPDcalc /KC )PDcalc = 0.217,KPDmeas = 0.2091,KC = 0.2103
Optical Data: Uniaxial (+), w=1.99, e=1.85, bire=0.1400.
Calculated Properties of Keystoneite
Electron Density: Bulk Density (Electron Density)=3.99 gm/cc
Fermion Index: Fermion Index = 0.04
Photoelectric: PEKeystoneite = 182.30 barns/electronU=PEKeystoneite x r Electron Density= 727.27 barns/cc.
Radioactivity: GRapi = 0 (Gamma Ray American Petroleum Institute Units)Not Radioactive
Keystoneite Classification
Dana Class: 34.03.02.03 (34) Selenites - Tellurites - Sulfites
(34.03) Dana Type
(34.03.02) Zemannite Group
34.03.02.01 Zemannite Mg0.5[ZnFe(TeO3)3]•4.5(H2O) P 63 /m 6/m
34.03.02.02 Kinichilite Mg0.5[MnFe(TeO3)3]•4.5(H2O) P 63 /m 6/m
34.03.02.03 Keystoneite Mg0.5[NiFe(TeO3)3]•4.5(H2O) P 63 /m 6/m
Strunz Class: 04.JM.05 04 - OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodate
04.J - Arsenites, Antimonites, Bismuthites, Sulfites,
04.JM -Tellurites without additional anions, with H2O
04.JM.05 Keystoneite Mg0.5[NiFe(TeO3)3]•4.5(H2O) P 63 /m 6/m
04.JM.05 Kinichilite Mg0.5[MnFe(TeO3)3]•4.5(H2O) P 63 /m 6/m
04.JM.05 Zemannite Mg0.5[ZnFe(TeO3)3]•4.5(H2O) P 63 /m 6/m
Other Keystoneite Information
References: NAME( Dana8) PHYS. PROP.(37th List of New Min.,Min. Mag.,1996) OPTIC PROP.(Dana8)
See Also: Links to other databases for Keystoneite : Am. Min. Crystal Structure Database Athena EUROmin Project Ecole des Mines de Paris GeoScienceWorld Google Images Google Scholar Handbook of Mineralogy (MinSocAm) Handbook of Mineralogy (UofA) MinDAT Mineralienatlas (Deutsch) Online Mineral Museum QUT Mineral Atlas Ruff.Info Search for Keystoneite using:
[AOL ]
[Bing ]
[Dog Pile ]
[GeoScienceWorld ]
[HotBot ]
[Ixquick ]
[Lycos ]
[MAMMA ]
[Scirus ]
[Teoma ]
[WebCrawler ]
[Wikipedia ]
[YAHOO ]
Visit our Advertisers for Keystoneite :
Ask about Keystoneite here : Ask-A-Mineralogist from the Mineralogical Society of AmericaMindat.org's Discussion GroupsOriginal Rockhounds Discussion GroupRockhounds Discussion Group on Yahoo GroupsMineral Discussion Forum from Fabre Minerals - also available in
Espaņol
Print or Cut-and-Paste your Keystoneite Specimen Label here :
Keystoneite
Mg0.5[Ni++Fe++(TeO3)3]•4.5(H2O) Dana No: 34.03.02.03 Strunz No: 04.JM.05 Locality:
Notes:
Dakota Matrix
Excalibur Minerals
Exceptional Minerals
Hudson Insitute
John Betts Fine Minerals
Mc Dougall Minerals
Mineral News
Rock and Mineral Shows
Weinrich Minerals, Inc.