Dickinsonite Mineral Data
General Dickinsonite Information
Chemical Formula: KNa4Ca(Mn++,Fe++)14Al(PO4)12(OH)2
Composition: Molecular Weight = 2,132.64 gm
Potassium 0.92 % K 1.10 % K2 O
Sodium 5.71 % Na 7.70 % Na2 O
Lithium 0.07 % Li 0.14 % Li2 O
Calcium 1.69 % Ca 2.37 % CaO
Manganese 24.73 % Mn 31.93 % MnO
Aluminum 1.01 % Al 1.91 % Al2 O3
Iron 10.21 % Fe 13.14 % FeO
Phosphorus 17.14 % P 39.27 % P2 O5
Hydrogen 0.18 % H 1.65 % H2 O
Oxygen 38.34 % O
______ ______
100.00 % 99.21 % = TOTAL OXIDE
Empirical Formula: K0.5 Li0.2 Na5.3 Ca0.9 Mn2+ 9.6 Fe2+ 3.9 Al0.8 (PO4 )11.8 (OH)3.9
Environment: High temperature (800 deg C) primary mineral in granite pegmatites.
IMA Status: Discredited IMA 2005 - Dana # Changed from 41.7.2.2
Locality: Branchville, Fairfield County, Connecticut, USA. Link to MinDat.org Location Data.
Name Origin: Named for Reverend John William Dickinson (1835-1899), Redding, Connecticut, USA, an early collector of Branchville minerals. Dickinsonite is now the group name.
Name Pronunciation: Dickinsonite + Pronunciation
Synonym: ICSD 100870
PDF 43-1466
Dickinsonite Image
Images:
Dickinsonite Crystallography
Axial Ratios: a:b:c =1.6783:1:2.4814
Cell Dimensions: a = 16.7, b = 9.95, c = 24.69, Z = 4; beta = 104.783° V = 3,966.81 Den(Calc)= 3.57
Crystal System: Monoclinic - Domatic
X Ray Diffraction: By Intensity(I/Io ): 3.05(1), 2.72(0.9), 3.22(0.7),
Forms:
Mouse
Dbl Clk - Start-Stop Rotation Keyboard
S - Stereo
Help on Above
Forms:
( 1 0 2 ) ( 1 1 0) ( 1 0 2) ( 1 1 1 ) ( 1 0 0) ( 0 0 1) Warning: this java applet does not work in some browsers because of security restrictions.
Physical Properties of Dickinsonite
Cleavage: {???} Perfect
Color: Brownish green, Green, Yellow green.
Density: 3.34
Diaphaneity: Transparent to Translucent
Fracture: Brittle - Uneven - Very brittle fracture producing uneven fragments.
Habit: Foliated - Two dimensional platy forms.
Habit: Micaceous - Platy texture with "flexible" plates.
Hardness: 3.5-4 - Copper Penny-Fluorite
Luminescence: Non-fluorescent.
Luster: Vitreous - Pearly
Streak: white
Optical Properties of Dickinsonite
Gladstone-Dale: CI meas = -0.032 (Excellent) - where the CI = (1-KPDmeas /KC ) calc = 0.034 (Excellent) - where the CI = (1-KPDcalc /KC )PDcalc = 0.1847,KPDmeas = 0.1974,KC = 0.1912
Optical Data: Biaxial (+), a=1.648-1.658, b=1.655-1.662, g=1.662-1.671, bire=0.0130-0.0140, 2V(Calc)=68-88, 2V(Meas)=80-90. Dispersion relatively strong.
Pleochroism (x): pale olive green.
Pleochroism (y): paler olive green.
Pleochroism (z): very pale yellow green.
Calculated Properties of Dickinsonite
Electron Density: Bulk Density (Electron Density)=3.22 gm/cc
Fermion Index: Fermion Index = 0.0024221687
Photoelectric: PEDickinsonite = 10.68 barns/electronU=PEDickinsonite x r electron = 34.38 barns/cc.
Radioactivity: GRapi = 12.76 (Gamma Ray American Petroleum Institute Units)
Concentration of Dickinsonite per GRapi unit = 7.84 (%)
Estimated Radioactivity from Dickinsonite
Dickinsonite Classification
Dana Class: 41.07.02d.00 (41) Anhydrous Phosphates, etc. Containing Hydroxyl or Halogen
(41.07) where (A B)m (XO4)4 Zq
(41.07.02d) Arrojadite Group (Ferri-arrojadite Subgroup) (Fe in Al site, OH in W site, Fe in M site)
41.07.02d.00 Dickinsonite ? KNa4Ca(Mn,Fe)14Al(PO4)12(OH)2 Cc m
41.07.02d.01 Dickinsonite-(KMnNa) ! KNaMnNa3Ca(Mn,Fe,Mg)13Al(PO4)11(PO4)(OH,F)2 Cc m
41.07.02d.02 Dickinsonite-(KNaNa) ! KNaNa4Ca(Mn,Fe,Mg)13Al(PO4)11(PO4)(OH,F)2 Cc m
41.07.02d.03 Dickinsonite-(KNa) ! KNa4Ca(Mn,Fe,Mg)13Al(PO4)11(PO4)(OH,F)2 Cc m
41.07.02d.04 Dickinsonite-(NaNa) ! Na2Na4Ca(Mn,Fe,Mg)13Al(PO4)11(PO4)(OH,F)2 Cc m
Strunz Class: 08.BF.05 08 - PHOSPHATES, ARSENATES, VANADATES
08.B - Phosphates, etc. with Additional Anions, without H2O
08.BF -With medium-sized and large cations, (OH, etc.):RO4 < 0.5:1
08.BF.05 Arrojadite ! KNa4CaMn4Fe10Al(PO4)12(OH,F)2 Cc m
08.BF.05 Dickinsonite ! KNa4Ca(Mn,Fe)14Al(PO4)12(OH)2 Cc m
08.BF.05 Arrojadite-(BaFe) ! (Ba,K,Pb)Na3(Ca,Sr)(Fe,Mg,Mn)14Al(PO4)11(PO3OH)(OH,F)2 Cc m
08.BF.05 Arrojadite-(KFe) ! KNa2CaNa2(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(OH,F)2 Cc m
08.BF.05 Arrojadite-(NaFe) ! NaNa2CaNa2(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(OH,F)2 Cc m
08.BF.05 Arrojadite-(SrFe) ! SrFeNa2Ca(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(OH,F)2 Cc m
08.BF.05 Arrojadite-(KNa) ! KNa4Ca(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(OH,F)2 Cc m
08.BF.05 Dickinsonite-(KNa) ! KNa4Ca(Mn,Fe,Mg)13Al(PO4)11(PO4)(OH,F)2 Cc m
08.BF.05 Arrojadite-(PbFe) ! PbFeNa2Ca(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(OH,F)2 Cc m
08.BF.05 Fluorarrojadite-(BaNa) ! BaFeNa2Ca(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(F,OH)2 Cc m
08.BF.05 Arrojadite-(BaNa) ! BaFeNa2Ca(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(OH,F)2 Cc m
08.BF.05 Fluorarrojadite-(KNa) ! KNa4Ca(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(F,OH)2 Cc m
08.BF.05 Fluorarrojadite-(BaFe) ! (Ba,K,Pb)Na3(Ca,Sr)(Fe,Mg,Mn)14Al(PO4)11(PO3OH)(F,OH)2 Cc m
08.BF.05 Ferri-arrojadite-(BaNa) ! BaFeNa2Ca(Fe,Mn,Mg)13Al(PO4)11(PO3OH)(F,OH)2 Cc m
08.BF.05 Dickinsonite-(KMnNa) ! KNaMnNa3Ca(Mn,Fe,Mg)13Al(PO4)11(PO4)(OH,F)2 Cc m
08.BF.05 Dickinsonite-(KNaNa) ! KNaNa4Ca(Mn,Fe,Mg)13Al(PO4)11(PO4)(OH,F)2 Cc m
08.BF.05 Dickinsonite-(NaNa) ! Na2Na4Ca(Mn,Fe,Mg)13Al(PO4)11(PO4)(OH,F)2 Cc m
Other Dickinsonite Information
References: NAME( AntBidBlaNic4) PHYS. PROP.(Enc. of Minerals,2nd ed.,1990) OPTIC PROP.(AntBidBlaNic4)
See Also: Links to other databases for Dickinsonite : Am. Min. Crystal Structure Database Athena EUROmin Project Ecole des Mines de Paris GeoScienceWorld Google Images Google Scholar Handbook of Mineralogy (MinSocAm) Handbook of Mineralogy (UofA) MinDAT Mineralienatlas (Deutsch) Online Mineral Museum QUT Mineral Atlas Ruff.Info WWW-MINCRYST Search for Dickinsonite using:
[AOL ]
[Bing ]
[Dog Pile ]
[GeoScienceWorld ]
[HotBot ]
[Ixquick ]
[Lycos ]
[MAMMA ]
[Scirus ]
[Teoma ]
[WebCrawler ]
[Wikipedia ]
[YAHOO ]
Visit our Advertisers for Dickinsonite :
Ask about Dickinsonite here : Ask-A-Mineralogist from the Mineralogical Society of AmericaMindat.org's Discussion GroupsOriginal Rockhounds Discussion GroupRockhounds Discussion Group on Yahoo GroupsMineral Discussion Forum from Fabre Minerals - also available in
Espaņol
Print or Cut-and-Paste your Dickinsonite Specimen Label here :
Dickinsonite
KNa4Ca(Mn++,Fe++)14Al(PO4)12(OH)2 Dana No: 41.07.02d.00 Strunz No: 08.BF.05 Locality:
Notes:
Dakota Matrix
Excalibur Minerals
Exceptional Minerals
Hudson Insitute
John Betts Fine Minerals
Mc Dougall Minerals
Mineral News
Rock and Mineral Shows
Weinrich Minerals, Inc.



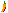 - barely detectable
- barely detectable