Alum-(K) Mineral Data
General Alum-(K) Information
Chemical Formula: KAl(SO4)2•12(H2O)
Composition: Molecular Weight = 474.39 gm
Potassium 8.24 % K 9.93 % K2 O
Aluminum 5.69 % Al 10.75 % Al2 O3
Hydrogen 5.10 % H 45.57 % H2 O
Sulfur 13.52 % S 33.75 % SO3
Oxygen 67.45 % O
______ ______
100.00 % 100.00 % = TOTAL OXIDE
Empirical Formula: KAl(SO4 )2 •12(H2 O)
Environment: Derived from the oxidation of sulfide minerals and potassium-bearing minerals.
IMA Status: Valid Species (Pre-IMA) 1875
Locality: Vesuvius, Italy. Alum Cave, Tennessee, USA. Link to MinDat.org Location Data.
Name Origin: Named for the element K and the Latin, alumen.
Synonym: Native Alum
Potash Alum
Potassium-alum - Renamed to Alum-(K) by Mineralogical Record, v39 (2008), p131
Alum-(K) Image
Images:
Alum-(K)
Comments: White encrustations and ballshaped aggregates of potassium alum.Location: Scholler mine, Libusin, Kladno, Czech Republic.
Scale: See Fingers for Scale.© Diederik Visser
Alum-(K) Crystallography
Cell Dimensions: a = 12.133, Z = 4; V = 1,786.10 Den(Calc)= 1.76
Crystal System: Isometric - Diploidal 3 ) Space Group: P a3
X Ray Diffraction: By Intensity(I/Io ): 4.298(1), 3.25(0.55), 4.053(0.45),
Physical Properties of Alum-(K)
Cleavage: {111} Indistinct
Color: Colorless, White.
Density: 1.76
Diaphaneity: Transparent
Fracture: Conchoidal - Fractures developed in brittle materials characterized by smoothly curving surfaces, (e.g. quartz).
Habit: Water Soluble - Water soluble mineral.
Hardness: 2 - Gypsum
Luminescence: Non-fluorescent.
Luster: Vitreous (Glassy)
Streak: white
Optical Properties of Alum-(K)
Gladstone-Dale: CI meas = -0.004 (Superior) - where the CI = (1-KPDmeas /KC ) calc = -0.004 (Superior) - where the CI = (1-KPDcalc /KC )PDcalc = 0.2574,KPDmeas = 0.2574,KC = 0.2564
Optical Data: Isotropic, n=1.453.
Calculated Properties of Alum-(K)
Electron Density: Bulk Density (Electron Density)=1.84 gm/cc
Fermion Index: Fermion Index = 0.0013552227
Photoelectric: PEAlum-(K) = 1.89 barns/electronU=PEAlum-(K) x r electron = 3.47 barns/cc.
Radioactivity: GRapi = 124.32 (Gamma Ray American Petroleum Institute Units)
Concentration of Alum-(K) per GRapi unit = 0.80 (%)
Estimated Radioactivity from Alum-(K)
Alum-(K) Classification
Dana Class: 29.05.05.01 (29) Hydrated Acid and Sulfates
(29.05) where A B (XO4)2 � x(H2O)
(29.05.05) Alum Group
29.05.05.01 Alum-(K) KAl(SO4)2•12(H2O) P a3 2/m 3
29.05.05.02 Alum-(Na) NaAl(SO4)2•12(H2O) P a3 2/m 3
29.05.05.03 Tschermigite (NH4)Al(SO4)2•12(H2O) P a3 2/m 3
29.05.05.04 Lonecreekite (NH4)(Fe,Al)(SO4)2•12(H2O) P a3 2/m 3
29.05.05.05 Lanmuchangite ! TlAl(SO4)2•12(H2O) P a3 2/m 3
Strunz Class: 07.CC.20 07 - SULFATES (SELENATES, TELLURATES)
07.C - Sulfates (selenates, etc.) without Additional Anions, with H2O
07.CC -With medium-sized and large cations
07.CC.20 Lonecreekite (NH4)(Fe,Al)(SO4)2•12(H2O) P a3 2/m 3
07.CC.20 Alum-(K) KAl(SO4)2•12(H2O) P a3 2/m 3
07.CC.20 Alum-(Na) NaAl(SO4)2•12(H2O) P a3 2/m 3
07.CC.20 Lanmuchangite ! TlAl(SO4)2•12(H2O) P a3 2/m 3
07.CC.20 Tschermigite (NH4)Al(SO4)2•12(H2O) P a3 2/m 3
Other Alum-(K) Information
References: NAME( Dana7) PHYS. PROP.(Enc. of Minerals,2nd ed.,1990) OPTIC PROP.(Dana7)
See Also: Links to other databases for Alum-(K) : Am. Min. Crystal Structure Database Athena GeoScienceWorld Google Images Google Scholar MinDAT Mineralienatlas (Deutsch) Online Mineral Museum QUT Mineral Atlas Ruff.Info Search for Alum-(K) using:
[AOL ]
[Bing ]
[Dog Pile ]
[GeoScienceWorld ]
[HotBot ]
[Ixquick ]
[Lycos ]
[MAMMA ]
[Scirus ]
[Teoma ]
[WebCrawler ]
[Wikipedia ]
[YAHOO ]
Visit our Advertisers for Alum-(K) :
Ask about Alum-(K) here : Ask-A-Mineralogist from the Mineralogical Society of AmericaMindat.org's Discussion GroupsOriginal Rockhounds Discussion GroupRockhounds Discussion Group on Yahoo GroupsMineral Discussion Forum from Fabre Minerals - also available in
Español
Print or Cut-and-Paste your Alum-(K) Specimen Label here :
Alum-(K)
KAl(SO4)2•12(H2O) Dana No: 29.05.05.01 Strunz No: 07.CC.20 Locality:
Notes:
Dakota Matrix
Excalibur Minerals
Exceptional Minerals
Hudson Insitute
John Betts Fine Minerals
Mc Dougall Minerals
Mineral News
Rock and Mineral Shows
Weinrich Minerals, Inc.


Small.jpg)
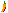 - barely detectable
- barely detectable